1. HIV epidemic in Belgium
i. Trends in HIV diagnoses
After the decreasing trend in the number of new HIV diagnoses between 2012 and 2018, an epidemiological plateau was reached in 2019. Between 2019 and 2020, the number of new HIV diagnoses decreased by 21%. This sharp decline was clearly related to the COVID-19 pandemic and the measures taken to curb the circulation of COVID-19 virus. Both had an impact on HIV testing activities, sexual behaviour and migration dynamics.
In 2020, there were 727 new HIV diagnoses, which corresponds to 2 new diagnoses per day. Belgium remains one of the Western European countries with proportionally the highest number of new HIV diagnoses (6.3/100 000 inhabitants)1.
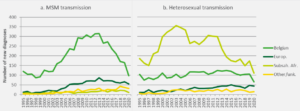
Since the start of the HIV epidemic in Belgium, two key populations have been particularly affected, namely men who have sex with men (MSM), of Belgian nationality, and heterosexual men and women from Sub-Saharan Africa. Given the declining trend of diagnoses in these key populations, the proportion of populations with other profiles has become relatively larger in recent years and the epidemic in Belgium is now more diversified. In 2020, 40% of the new HIV diagnoses were among Belgian men who have sex with men and heterosexuals from Sub-Saharan Africa.
The decreasing trend in the number of new HIV diagnoses among Belgian men who have sex with men continued in 2020; there was a slight increase among men who have sex with men from Latin America. Among heterosexuals of sub-Saharan African origin, the downward trend seemed to have stopped in 2019, but it resumed in 2020; there was also a decrease among heterosexuals with Belgian nationality (figure 1).
Figure 1: Evolution of the number of new HIV diagnoses by transmission mode and nationality, Belgium 1995-2020
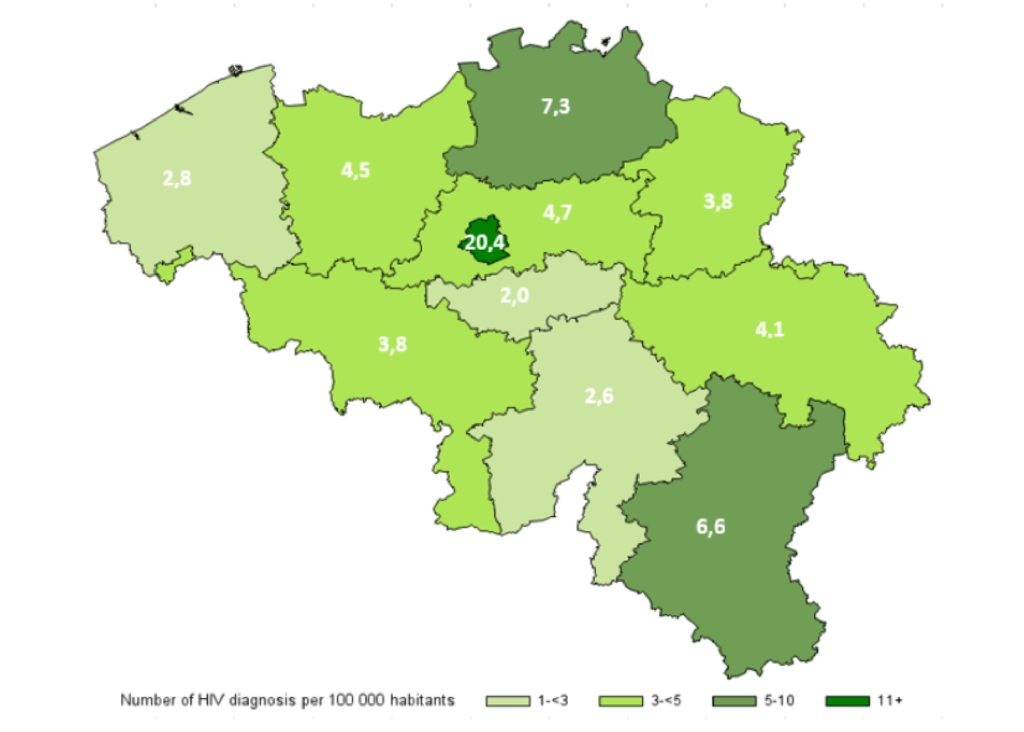
In terms of geographical distribution, the highest number of HIV diagnoses in 2020 was reported in the Brussels Capital Region with 20.4 new diagnoses per 100 000 inhabitants (figure 2). The number of diagnoses was higher in cities (12.3 diagnoses per 100 000 inhabitants) than in agglomerations and suburbs (3.5 per 100 000 inhabitants) or in sparsely populated areas (2.6 per 100 000 inhabitants). The higher number of diagnoses in Brussels therefore seems to be linked, on the one hand, to the urban nature of the region and, on the other, to the specific characteristics of the capital.
Figure 2: Number of HIV diagnoses per 100 000 inhabitants and per province and Brussels Capital Region, 2020
ii. Testing and diagnosis process
Late diagnosis of HIV infection remains a challenge as an HIV infection does not necessarily lead to an immediate diagnosis. The timing of the diagnosis is influenced by various factors such as the slow progression of the disease, as well as the availability and frequency of test activities. In 2020, 36% of HIV infections were diagnosed late (<350 CD4 /mm³ at the time of the HIV diagnosis). The number of late diagnoses has moderately decreased in the recent years and it remains more common among heterosexuals (47%) than among men who have sex with men (22%).
In 2020, half of the HIV tests in Belgium were carried out by general practitioners through whom 42% of the HIV diagnoses are made.
iii. Estimates of people living with HIV: diagnosed and undiagnosed
In 2020, 18 753 people were estimated to be living with HIV, this represents a prevalence of 1.7/1000 inhabitants. Recent surveys among most-affected populations reported HIV prevalence among migrants from sub-Saharan Africa of 5.9% for women and 4.2% for men2. Among men who have sex with men, reported HIV prevalence is about 12% in both the Sialon survey (based on biological sampling) in Brussels3 and the EMIS online survey (based on self-reported data) in Belgium4.
People living with HIV (PLWH) may remain unaware of their HIV status for a considerable time before diagnosis. HIV-infected people who are unaware of living with HIV cannot benefit from highly effective treatment and may unwillingly contribute to the on-going transmission of HIV infection. In 2020, an estimated 1585 were not aware of their infection. The estimated number and proportion of people living with HIV that was not aware of their serostatus decreased from 23.5% of people living with HIV in 2008 to 8.5% in 2020. Geographic areas hosting the biggest cities in Belgium accounted for the vast majority of undiagnosed HIV infections and individuals with foreign nationality were the most affected, especially men who have sex with men with non‐European nationality5.
iv. Care of people living with HIV
As a result of new infections and low mortality, the number of people living with HIV increases by an average 646 patients per year. Exceptionally in 2020, there was a slight decrease in the number of patients in medical follow-up due to the COVID-19 pandemic. In 2020, 17 018 patients were followed up for HIV care in Belgium. An increasing proportion of patients become elderly: from 19% of patients aged 50 years or more in 2006 to 44% in 2020. In parallel, patients have a longer average duration since infection and since ART initiation and are therefore at higher risk for (multi)comorbidities.
Clinical care indicators for the patients followed in the HIV Reference Centres (HRCs) are very good: ART coverage was 75% in 2008 and increased to 98% of the patients in 2020, and controlled viral load (<200 copies/mL) reached 98% of those on ART for at least 6 months.
Optimal care for HIV patients requires a continuity of services. From a clinical and public health perspective, early and sustained HIV care and treatment are associated with viral suppression, improved health outcomes and reductions in transmission risks. The following risk factors related to poorer retention in HIV care in Belgium were identified: being younger, using injecting drugs, being diagnosed recently and not being Belgian, whilst men who have sex with men had higher retention rates6.
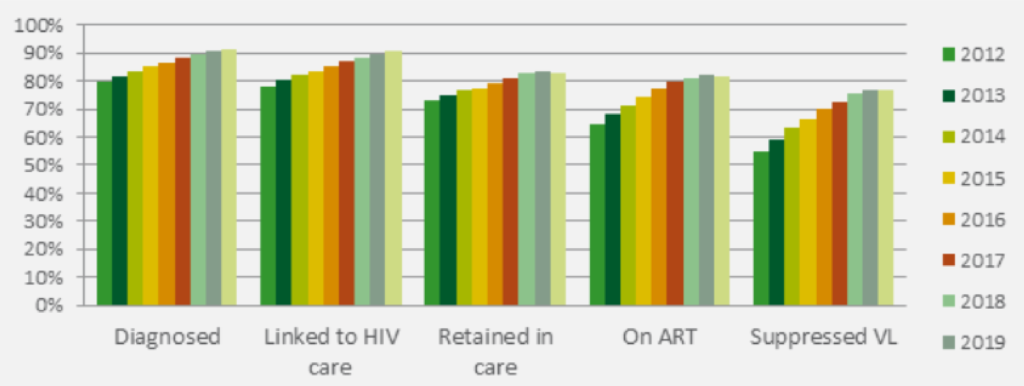
The continuum of HIV care is a monitoring framework that uses cross-sectional indicators to quantify the number of people diagnosed, linked to care, retained in care, on ART, and achieving a controlled viral load, as a proportion of the estimated number of people living with HIV. In 2020, of all people living with HIV in Belgium 92% were diagnosed, 90% had been linked to HIV care, 83% were retained in care, 82% were receiving ART and 77% were virally supressed (figure 3). Improvement in all stages of the continuum was observed over the years and particularly for the ART uptake and viral load suppression.
Figure 3: Continuum of HIV care in Belgium, 2012-2020
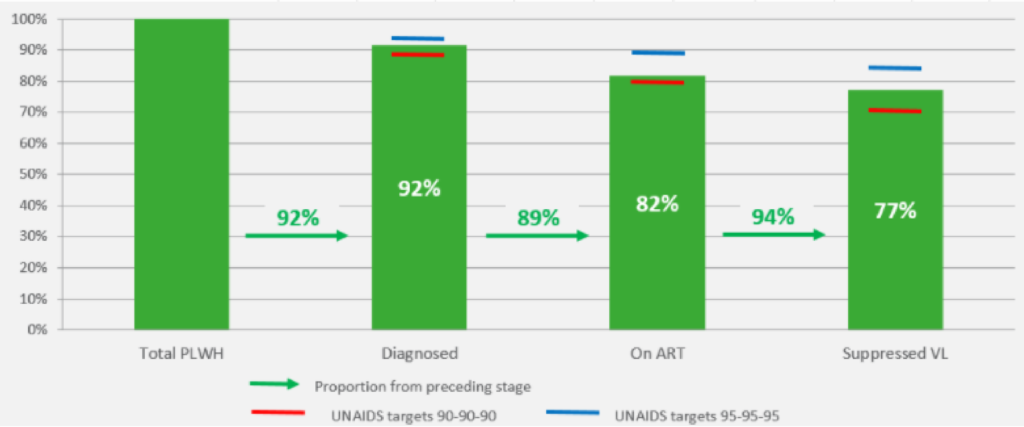
For 2020, UNAIDS has set the ambitious 90-90-90 global target: 90% of people living with HIV should know their positive serostatus, 90% of these should receive antiretroviral treatment and of these 90% should have an undetectable viral load. Once this triple target is met, the overall 2020 goal of at least 73% of people living with HIV having an undetectable viral load will have been achieved. Belgium has achieved this overall target: in 2020, 92% of the HIV population was diagnosed, 89% of them received antiretroviral treatment and 94% of them had an undetectable viral load, or 77% of all persons living with HIV (figure 4). Additional efforts to reduce new infections, ensure early diagnosis and retention in care will be decisive in order to achieve the next targets of 95-95-95 in 2030.
Figure 4: Continuum of HIV care in Belgium in 2020 compared to the UNAIDS targets
2. Related STI epidemics
i. Epidemiological and behavioural dynamics
Recent ECDC expert guidance states that the integration of an STI prevention and control strategy within an HIV strategy is the most logical and advantageous model of practice7. Additionally, integration with a viral hepatitis strategy is recommended as a supplementary option8.
The case for integration is strengthened by the common modes of transmission, leading to overlaps in the affected key populations. At the individual level this results in co-infections. The presence of an STI makes individuals more vulnerable for an HIV infection and being both infected with HIV and another STI may increase the risk of further transmission of HIV. At population level, HIV and STI epidemics are fuelled by similar epidemiological dynamics, the so called syndemics9. By joining prevention and testing efforts, a synergistic approach can be used to combat HIV and STI more effectively and efficiently.
However, not all most-affected populations for HIV are at equal risk of STI. In Belgium, as in most Western countries, the observed overall increase of syphilis and gonorrhoea diagnoses since 2002 has been predominantly due to transmission between men who have sex with men10 11.
Chlamydia, gonorrhoea and syphilis are highly prevalent among certain subgroups of men who have sex with men. At the behavioural level, this high STI prevalence is associated with less consistent condom use, having multiple sex partners and substance use or dependence. LGV (Chlamydia serovar L) and sexually acquired hepatitis C are proportionally more diagnosed among HIV positive men who have sex with men. Infection with hepatitis C is related to specific high risk traumatic sexual practices, such as the practice of fisting12.
However, since the introduction of PrEP, these STI are also becoming more prevalent among HIV negative men who have sex with men on PrEP13 14.
STIs are far less prevalent among Sub-Saharan African migrants as compared to men who have sex with men. A recent study among Sub-Saharan African communities in Antwerp city revealed that STI diagnoses are associated with HIV infection.
ii. Reported cases 15
Chlamydia is the most common STI in Belgium. The number of reported cases in Belgium increased from 9.5/100 000 inhabitants in 2002 to 77.0/100 000 inhabitants in 2019. Nevertheless, this increase has been associated with more sensitive diagnostics and more targeted and opportunistic testing in risk groups. At European level, more cases are reported among heterosexuals, mainly women. Nevertheless, 10% of chlamydia cases are diagnosed among men who have sex with men16. Between 2014 and 2019, there was a steady increase in the number of LGV (Chlamydia serovar L) cases among men who have sex with men.
Gonorrhoea showed an increasing trend since 2002 from 2.6/100 000 inhabitants in 2002 to 26.0/100 000 inhabitants in 2019. In 2019, gonorrhoea was mainly registered among men between 20 and 39 years of age. At European level and in countries with a similar social and epidemiological context such as the Netherlands up to 50% of cases are found in men who have sex with men17.
Syphilis also increased over the same period (2002-2019) in Belgium from 0.4/100 000 inhabitants in 2002 to 21.6/100 000 inhabitants in 2019. In 2019, syphilis was mainly registered among men between 20 and 59 years old. European data on transmission of syphilis show that up to 75% of cases are diagnosed among men who have sex with men18.
3. Key populations
men who have sex with men and Sub-Saharan African migrants are the most-affected populations in the Belgian HIV epidemic. However, not all people belonging to these most-affected populations are at equal risk: HIV transmission dynamics depend on the influence of the various risk and vulnerability factors to which they are exposed and which act in a mutually reinforcing way.
That is why the HIV Plan focuses on key-populations defined as groups who, due to specific higher risk behaviour and contextual vulnerabilities are at increased risk of HIV and related STI. These populations are key to the HIV epidemic and key to the HIV response in Belgium and include men who have sex with men, (undocumented) migrants from high HIV-prevalence countries, transgender people, sex workers, people who inject drugs and prisoners. Within each key-population, young people have specific age-or developmentally related needs, which the Plan's specific actions will consider.
Addressing key-populations for HIV implies therefore addressing a combination of vulnerability factors such as inadequate access to health care, multi and intersectional discrimination, HIV-related stigma and socio-economic inequalities.
Despite the existence of support mechanisms to ensure universal health care, for example through the procedure of urgent medical care, complex administrative procedures may constitute important barriers to the actual accessibility of services. Also, Belgium provides a strong and progressive regulatory framework regarding discrimination that protects - among others - discrimination based on gender, ethnicity, sexual orientation, current and future health status19. Nevertheless, HIV-related perceived and real stigma and discrimination stemming from cultural practices, religious beliefs, legal restrictions and criminalization of HIV transmission continue to increase vulnerabilities to HIV infection. In addition to or intertwined with HIV, people living with HIV may face stigma and discrimination based on race, sexual orientation or gender identity. Discrimination and (self-)stigma may lead to not accessing care and self-exclusion from social support20.
Additionally, some key populations face poverty and experience limited access to essential services such as housing, education, employment, protection and justice21. These factors represent additional vulnerabilities in the sense that they undermine control over sexual health and increase the risk of sexual violence and practices of transactional sex. Also the linkages between migration, poverty and HIV have been recognised across the world. Research in Belgium has shown that HIV infection among sub-Saharan African migrants is associated with socio-economic vulnerability, next to concurrency and having sex mainly within African sexual networks22. It has also been found that Sub-Saharan Africans migrants are mobile and that they may engage in sexual risk behaviours while travelling, increasing as such the risk of post-migration HIV-acquisition23.
A number of good practices have shown that interventions, directly addressing key populations, can reach positive results. Within the Belgian context, harm reduction services have succeeded in keeping the impact of HIV among people who inject drugs relatively low compared to other countries. The efficient preventive approach towards – but also by – sex workers not only reduced the risk of HIV/STI infections, but also targeted other related risks such as violence. In addition, several community-based organisations have developed expertise in working within a participatory approach, thereby closely collaborating with the communities affected, increasing prevention demand and ownership for complementary services, such as peer support.
Summarizing, continuous interventions are needed to improve access to HIV prevention and care services. Comprehensive HIV prevention programs should address not only the biological drivers of HIV infection, but also the social context and structural factors that shape people’s sexual agency and health seeking behaviour. Effectively fighting HIV means defending human rights and combating all forms of discrimination as well as working within a multisector approach to address the socio-economic inequalities.
4. Mapping HIV stakeholders
i. HIV prevention & support organisations
Diverse organisations working on health promotion, prevention and support are part of the day-to-day implementation of activities towards key populations affected by HIV. These include organisations that provide one or more of the below services:
- general support towards key populations such as sex workers, LGBTI, migrants, people who inject drugs and prisoners;
- HIV/STI information and preventive services towards the general public or populations at increased risk of infection;
- structural advocacy to defend and protect the rights of those at risk of stigma and discrimination (socially/structurally);
- direct support to those affected/infected by HIV via peer-to-peer support, information and counselling.
ii. HIV Reference Centres
The Belgian HIV Reference Centres (HRC) are centres of expertise recognised by the government. An HRC is linked to a hospital, and provides accessible and multidisciplinary care for people with HIV, including medical treatment and care, psychosocial support and STI screening. The recognised HRC have a convention with the National Institute for Health and Disability Insurance (RIZIV/INAMI) which allows for the reimbursement of the medical treatment as well as the multidisciplinary care and support. Since 2017, the HRC are also in charge of PrEP service delivery. There are 12 recognised HRC in Belgium, with a geographical spread that ensures the accessibility of this specialized care. Five of these recognised HRC are located in Flanders (Antwerp, Leuven, Hasselt, Ghent, and Bruges), four in Brussels (UZ Brussels, St. Pierre, Erasmus, St. Luc) and three in Wallonia (Liège, Charleroi, Namur).
iii. AIDS Reference Laboratories
The Belgian Aids Reference Laboratories (ARL) are centres of expertise founded in 1987. They are working in close collaboration with HIV Reference Centres. There are three ARL in Flanders (Antwerp, Ghent and Leuven), three in Brussels (Jette, St Luc and Erasme) and one in Wallonia (Liège). Their tasks are to confirm or exclude HIV infection in case of a reactive screening test, to run analyses for the laboratory monitoring of HIV infection like viral load testing and drug resistance analysis; to evaluate the quality of tests used for diagnosis and follow-up of HIV infected patients and to document new infections in a way that allows epidemiologic analyses and scientific research. For the latter there is a close collaboration with Sciensano. The ARL are financed through a convention with RIZIV/INAMI.
iv. Low threshold screening centres
Based on Royal Decree from the 6th of March 2017 (previously the Royal Decree from the 28th of December 2006), RIZIV/INAMI finances three HRC as low threshold screening centres: Elisa Centre, HRC St Pierre; Sida Sol A.S.B.L, HRC Liège and Helpcenter, HRC Antwerp. The screening referred to in the context of this Royal Decree is intended for any person who is at increased risk of an HIV or STI infection and who belongs to one or more of the following target groups:
- Migrants from a country with an HIV prevalence that is at least 10 times higher than the HIV prevalence in Belgium;
- Men who have sex with men;
- Intravenous drug users and their partners;
- Sex workers and their partners;
- Prostitution customers and their partners;
- Persons who have unprotected sexual relations outside the context of a permanent relationship;
- Persons who have multiple partners or who have sex with a partner who has multiple partners.
These people can be offered a free (and anonymous) HIV test, supplemented if necessary with an STI screening. The screening activities may be offered outside the premises of the screening centre in order to improve accessibility for the target groups.
v. Sciensano
Sciensano, the Belgian Health Institure, is in charge of the epidemiological surveillance of HIV/AIDS in Belgium. To this end, Sciensano collects notifications of new HIV diagnoses from the ARL and of new AIDS cases from clinicians. Since 2006, Sciensano also collects individual routine data on HIV patients in medical follow-up from the HRC and ARL: this is the Belgian HIV Cohort. In 2018, the monitoring of PrEP and PEP users has been included in the HIV surveillance.
STI such as chlamydia, gonorrhea and syphilis as well as some other STI are monitored by Sciensano through different surveillance sources.
vi. BREACH
BREACH, the Belgium Research on AIDS and HIV Consortium, is a collaborative effort between the Belgian HIV Reference Centres, Aids Reference Laboratories, scientific research groups and interest organisations in the field of HIV infection and AIDS. BREACH aims to facilitate and support Belgian HIV research of all kinds (clinical trials, epidemiology, public health, basic and translational research, health economics) both at a national and at an international level and to increase its overall visibility. In this view, annual symposia and scientific meetings are organised. Within BREACH there is also a Public Health Working Group and a PrEP Task Force; both platforms provide opportunities for exchange and collaboration.
--------------------------------------------------
1 : Sasse A, Deblonde J, De Rouck M, Montourcy M, Van Beckhoven D. Epidemiology of AIDS and HIV infection in Belgium. Sciensano; 2020. Dutch version available through: https://doi.org/10.25608/5c9n-4t26; French version available through via: https://doi.org/10.25608/35mc-0z39
2 Loos J, Nöstlinger C, Vuylsteke B, Deblonde J, Ndungu M, Kint I, Manirankunda L, Reyniers T, Adobea D, Laga M, Colebunders R. First HIV prevalence estimates of a representative sample of adult sub-Saharan African migrants in a European city. Results of a community-based, cross-sectional study in Antwerp, Belgium. PloS one. 2017;12(4). Available at: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0174677
3 The Sialon II Project. Report on a Bio-behavioural Survey among MSM in 13 European cities. ISBN 978-88-98768-55-4 Cierre Grafica, 2016. Editors: Massimo Mirandola, Lorenzo Gios, Nigel Sherriff, Igor Toskin, Ulrich Marcus, Susanne Schink, Barbara Suligoi, Cinta Folch, Magdalena Rosińska. Available at : http://www.sialon.eu/data2/file/133_Sialon%20II_Report%20on%20a%20Bio-behavioural%20Survey%20among%20MSM%20in%2013%20European%20cities.pdf
4 Schmidt AJ, Weatherburn P, Hickson F. The EMIS-Network. EMIS 2017 – The European Men-Who-Have Sex-With-Men Internet Survey. Key findings from 50 countries. Stockholm: ECDC 2019
5 Marty L., Van Beckhoven D., Ost C., Deblonde J., Costagliola D., Sasse A., Supervie V. and the HERMETIC Study Group. Estimates of the HIV undiagnosed population in Belgium reveals higher prevalence for MSM with foreign nationality and for geographic areas hosting big cities. JIAS. 2019, 22:e25371. Available at : https://doi.org/10.1002/jia2.25371
6 Van Beckhoven D, Florence E, De Wit S, Wyndham-Thomas C, Sasse A, Van Oyen H, Macq J; Belgian Research on AIDS, HIV Consortium (BREACH). Incidence rate, predictors and outcomes of interruption of HIV care: nationwide results from the Belgian HIV cohort. HIV Med. 2020 Oct;21(9):557-566. doi: 10.1111/hiv.12901. Epub 2020 Jul 5. PMID: 32627351; PMCID: PMC7540395.
7 European Centre for Disease Prevention and Control. Developing a national strategy for the prevention and control of sexually transmitted infections. Stockholm: ECDC; 2019. Available through: https://www.ecdc.europa.eu/sites/default/files/documents/strategies-to-control-STIs.pdf
8 European Centre for Disease Prevention and Control. Public health guidance on HIV, Hepatitis B and C testing in the EU/EEA - an integrated approach. Stockholm: ECDC; 2018. Available through: https://www.ecdc.europa.eu/sites/default/files/documents/hiv-hep-guidance-brief-6-december.pdf
9 Singer M, Bulled N, Ostrach B, Mendenhall E. Syndemics and the biosocial conception of health. The Lancet. 2017 Mar 4;389(10072):941-50
10 European Centre for Disease Prevention and Control. Gonorrhoea. In: ECDC. Annual epidemiological report for 2017. Stockholm: ECDC; 2019.Available through: https://www.ecdc.europa.eu/sites/default/files/documents/gonorrhoea-annual-epidemiological-report-2017.pdf
11 European Centre for Disease Prevention and Control. Syphilis. In: ECDC. Annual epidemiological report for 2017. Stockholm: ECDC; 2019. Available through: https://www.ecdc.europa.eu/sites/default/files/documents/syphilis-annual-epidemiological-report-2017.pdf
12 Apers L, Vandenberghe W, De Wit S, Kabeya K, Callens S, Buyze J, et al. Risk factors for HCV acquisition among HIV-positive MSM in Belgium. JAIDS J Acquir Immune Defic Syndr. 2015;68(5):585–593.
13 Sasse A, Deblonde J, De Rouck M, Montourcy M, Van Beckhoven D. Epidemiology of AIDS and HIV infection in Belgium. Sciensano; 2019. Dutch version available through: https://doi.org/10.25608/5c9n-4t26; French version available through: https://doi.org/10.25608/k6sn-n789;
14 Vuylsteke, B., Reyniers, T., De Baetselier, I., Nöstlinger, C., Crucitti, T., Buyze, J., Kenyon, C., Wouters, K. and Laga, M., 2019. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: results of a prospective cohort measuring adherence, sexual behaviour and STI incidence. Journal of the International AIDS Society, 22(10), p.e25407
15 Vanden Berghe, W., De Baetselier, I., Van Cauteren, D., Sasse, A., Quoilin, S. Surveillances des infections sexuellement transmissibles. Données pour la période 2017-2019. Bruxelles, Belgique : Sciensano. Numéro de rapport : D/2020/14.440/85. Available through: https://www.sciensano.be/sites/default/files/report_sti_sciensano_1719_fr.pdf
16 European Centre for Disease Prevention and Control. Chlamydia infection. In: ECDC. Annual epidemiological report for 2017. Stockholm: ECDC; 2019. Available through: https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2017-chlamydia-infection.pdf
17 European Centre for Disease Prevention and Control. Gonorrhoea. In: ECDC. Annual epidemiological report for 2017. Stockholm: ECDC; 2019. Available through: https://www.ecdc.europa.eu/sites/default/files/documents/gonorrhoea-annual-epidemiological-report-2017.pdf
18 European Centre for Disease Prevention and Control. Syphilis. In: ECDC. Annual epidemiological report for 2017. Stockholm: ECDC; 2019. Available through: https://www.ecdc.europa.eu/sites/default/files/documents/syphilis-annual-epidemiological-report-2017.pdf
19 UNIA. The 19 grounds of discrimination. Available at: https://www.unia.be/en/grounds-of-discrimination/the-19-grounds-of-discrimination
20 Pezeril C. Lutte contre le SIDA et promotion de la santé sexuelle. Santé Conjuguée, 2019 n° 86. Available at: https://observatoire-sidasexualites.be/wp-content/uploads/SC-86-in-lgbt-def-pezeril.pdf
21 Gennotte et al. (2017). Oral presentation at the BEACH symposium 2017, Anderlecht; Belgium. Available at : http://www.breach-hiv.be/media/docs/BREACHSympo2017/AMASE,AFGennotteBreach3pourlesite.pdf
22 Loos J, Nöstlinger C, Vuylsteke B, Deblonde J, Ndungu M, Kint I, Manirankunda L, Reyniers T, Adobea D, Laga M, Colebunders R. First HIV prevalence estimates of a representative sample of adult sub-Saharan African migrants in a European city. Results of a community-based, cross-sectional study in Antwerp, Belgium. PloS one. 2017;12(4). Available at: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0174677
23 Dias S, Gama A, Loos J, Roxo L, Simões D, Nöstlinger C (2020) The role of mobility in sexual risk behaviour and HIV acquisition among sub-Saharan African migrants residing in two European cities. PLoS ONE 15(2):e0228584. https://doi.org/10.1371/journal.pone.0228584
